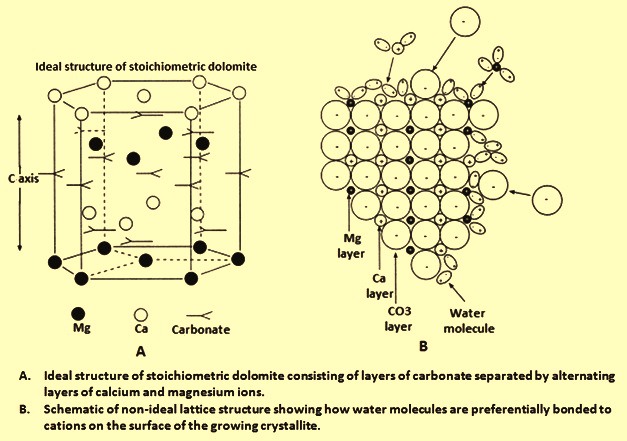
Solubility and Dissolution Kinetics of Dolomite in Ca–Mg–HCO3/CO3 Solutions at 25°C and 0.1 MPa Carbon Dioxide - Sherman - 2000 - Soil Science Society of America Journal - Wiley Online Library
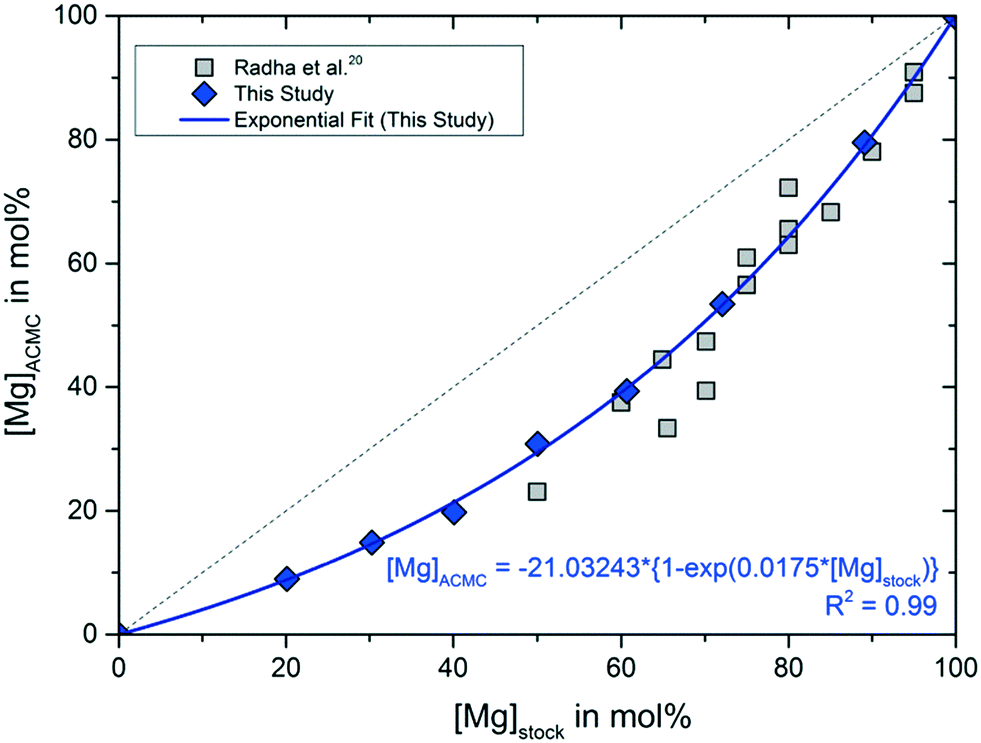
Solubility investigations in the amorphous calcium magnesium carbonate system - CrystEngComm (RSC Publishing) DOI:10.1039/C8CE01596A
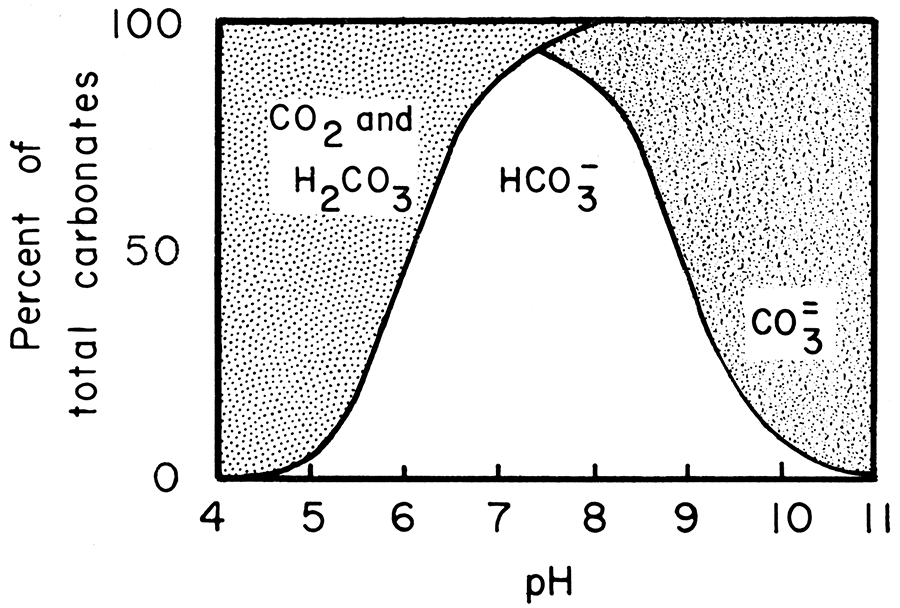
![The solubility (S) of dolomite [CaMg(CO 3 ) 2 ] as a function of pH... | Download Scientific Diagram The solubility (S) of dolomite [CaMg(CO 3 ) 2 ] as a function of pH... | Download Scientific Diagram](https://www.researchgate.net/profile/James-Altland/publication/309875485/figure/fig1/AS:537898228826112@1505256339484/The-solubility-S-of-dolomite-CaMgCO-3-2-as-a-function-of-pH-LlogH-D_Q640.jpg)
The solubility (S) of dolomite [CaMg(CO 3 ) 2 ] as a function of pH... | Download Scientific Diagram
Dolomite-Calcite Relationships in Sea Water: Theoretical Considerations and Preliminary Experimental Results

Solubility and Dissolution Kinetics of Dolomite in Ca–Mg–HCO3/CO3 Solutions at 25°C and 0.1 MPa Carbon Dioxide - Sherman - 2000 - Soil Science Society of America Journal - Wiley Online Library

The impact of Mg2+ ions on equilibration of Mg-Ca carbonates in groundwater and brines - ScienceDirect

Calcite, dolomite and magnesite dissolution kinetics in aqueous solutions at acid to circumneutral pH, 25 to 150 °C and 1 to 55 atm pCO2: New constraints on CO2 sequestration in sedimentary basins - ScienceDirect
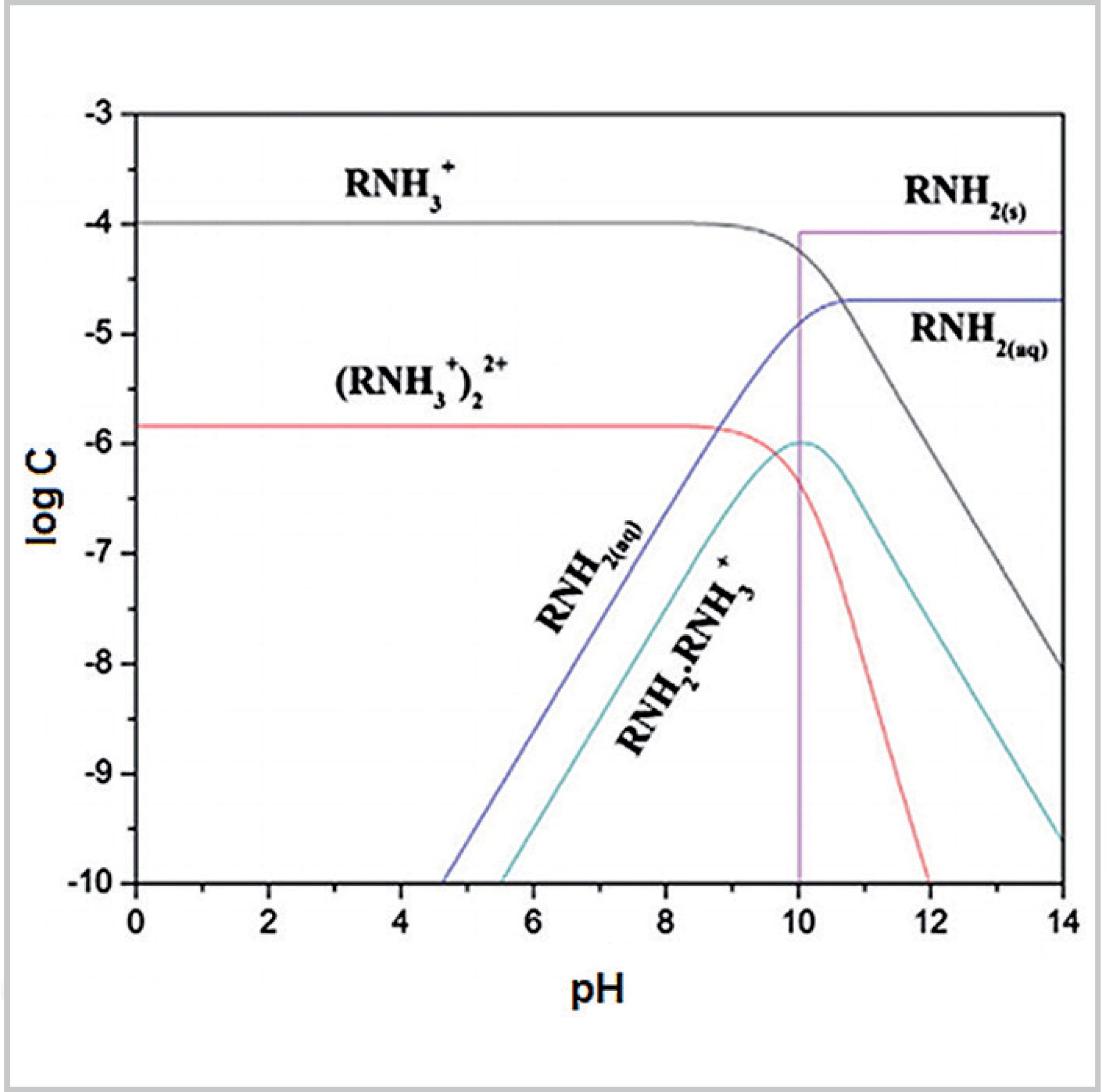
SciELO - Brasil - Influence of the pH regulator on the dolomite hydrophobization process Influence of the pH regulator on the dolomite hydrophobization process
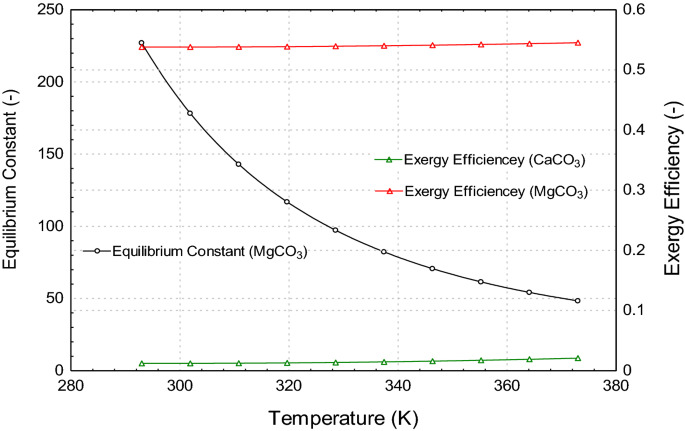
Thermodynamic analysis of theoretical dolomite formation from seawater and captured carbon dioxide | SpringerLink
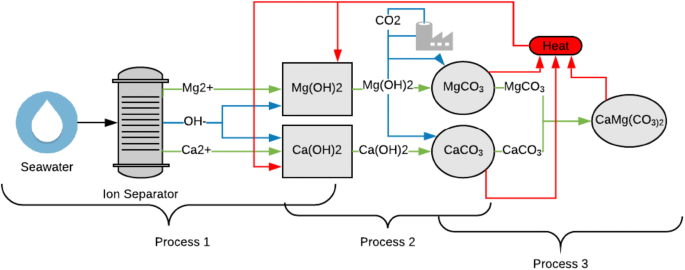
Thermodynamic analysis of theoretical dolomite formation from seawater and captured carbon dioxide | SpringerLink
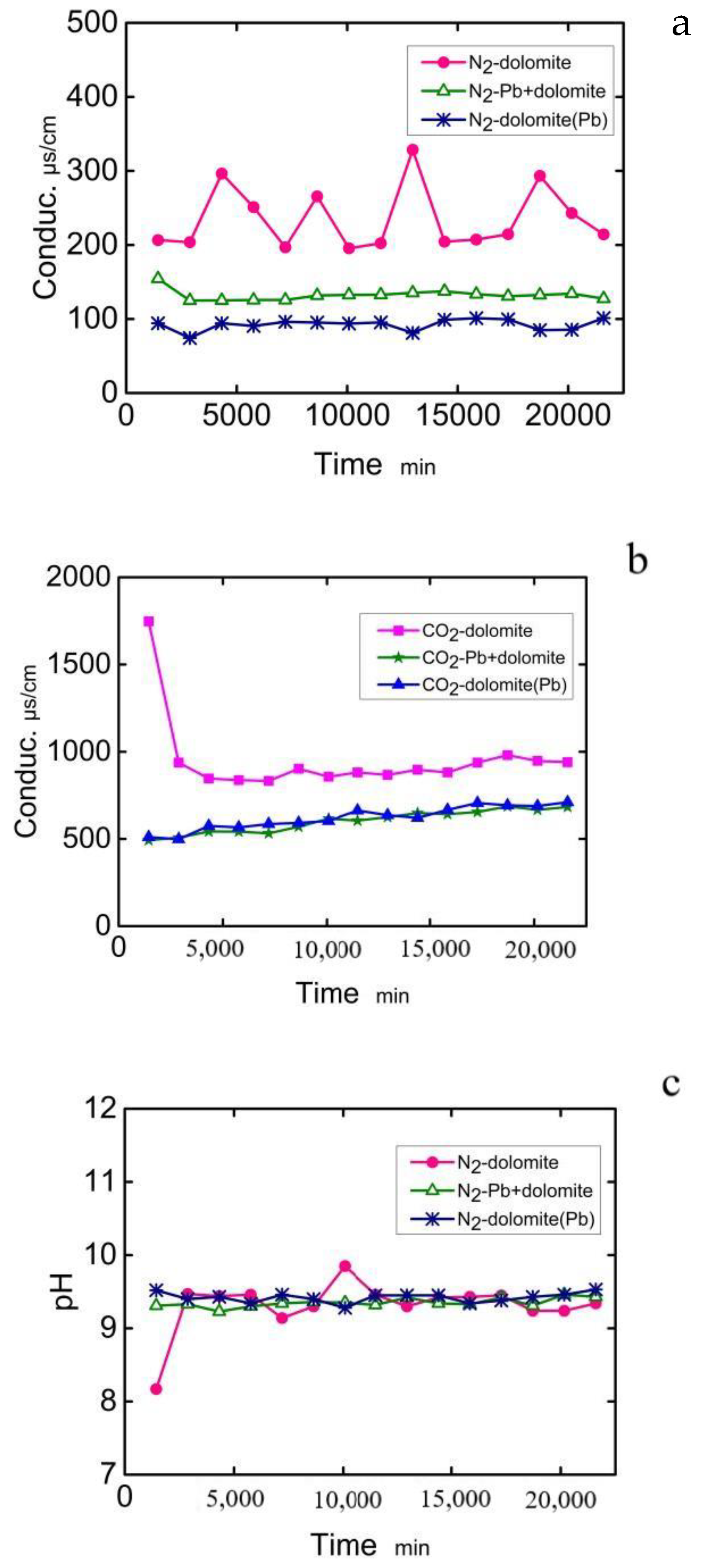
Water | Free Full-Text | Divalent Lead in Aqueous Solution Changes the Surface Morphology of Dolomite and Inhibits Dissolution | HTML

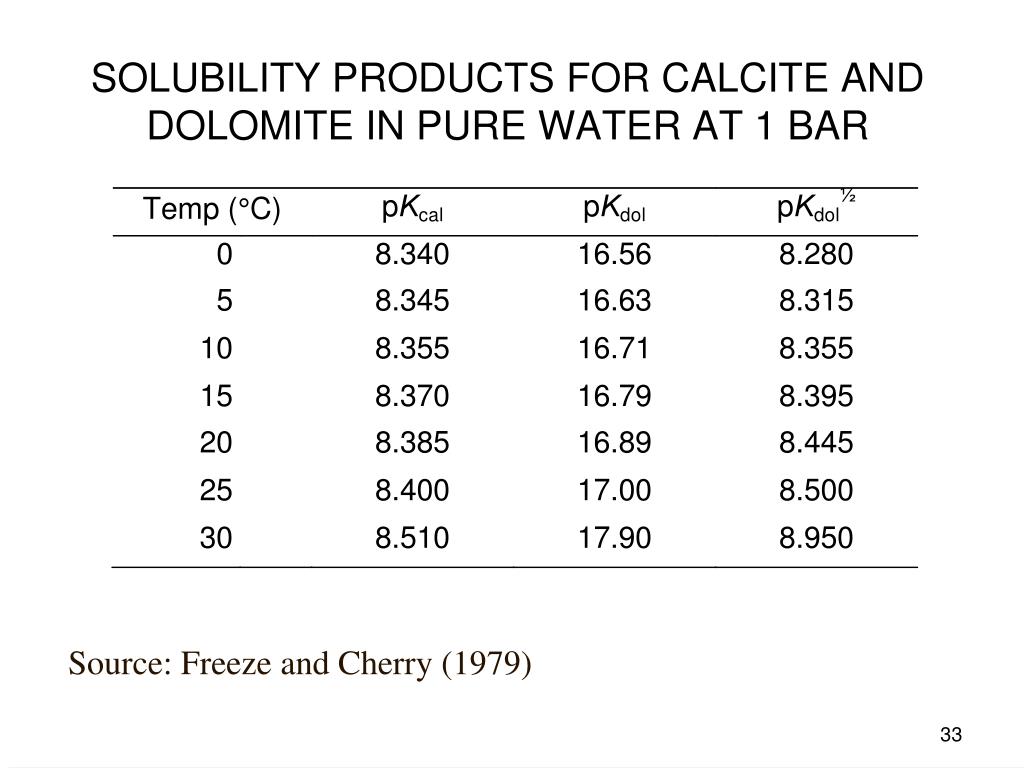
Solubility product constants for natural dolomite (0–200 °C) through a groundwater-based approach using the USGS produced water database | American Journal of Science
![The solubility (S) of dolomite [CaMg(CO 3 ) 2 ] as a function of pH... | Download Scientific Diagram The solubility (S) of dolomite [CaMg(CO 3 ) 2 ] as a function of pH... | Download Scientific Diagram](https://www.researchgate.net/publication/309875485/figure/fig1/AS:537898228826112@1505256339484/The-solubility-S-of-dolomite-CaMgCO-3-2-as-a-function-of-pH-LlogH-D.png)
The solubility (S) of dolomite [CaMg(CO 3 ) 2 ] as a function of pH... | Download Scientific Diagram

Solubility product constants for natural dolomite (0–200 °C) through a groundwater-based approach using the USGS produced water database | American Journal of Science

Solubility and Dissolution Kinetics of Dolomite in Ca–Mg–HCO3/CO3 Solutions at 25°C and 0.1 MPa Carbon Dioxide - Sherman - 2000 - Soil Science Society of America Journal - Wiley Online Library