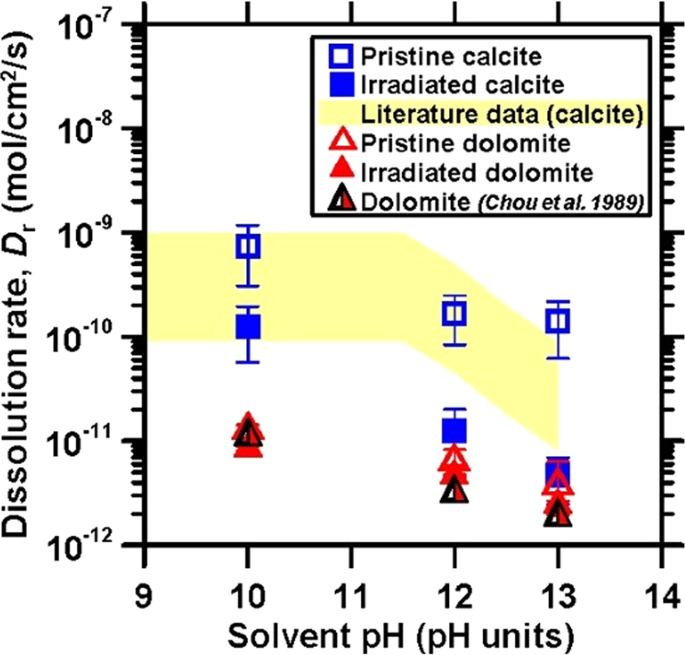
The effect of irradiation on the atomic structure and chemical durability of calcite and dolomite | npj Materials Degradation

An experimental study simulating the dissolution of gypsum rock - Dongdong Hong, Ming Fan, Lingjie Yu, Jian Cao, 2018

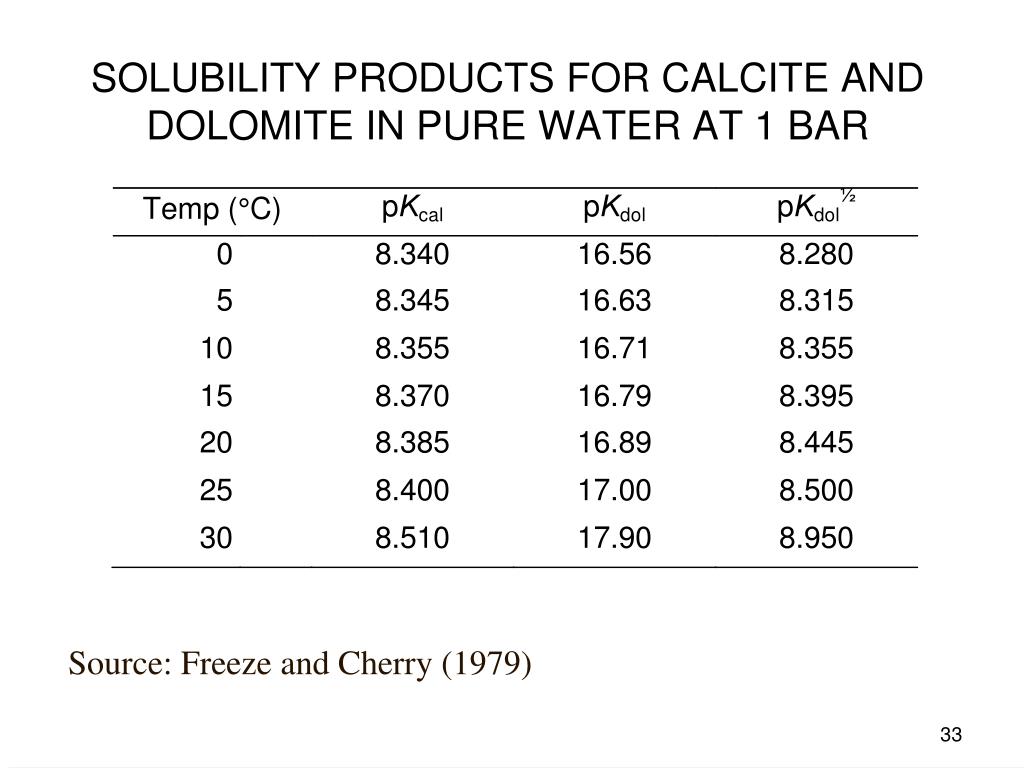
Solubility product constants for natural dolomite (0–200 °C) through a groundwater-based approach using the USGS produced water database | American Journal of Science

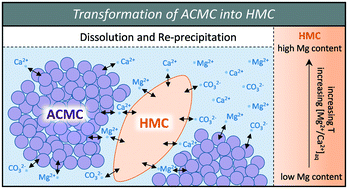
Low-Temperature Synthesis of Disordered Dolomite and High-Magnesium Calcite in Ethanol–Water Solutions: The Solvation Effect and Implications | ACS Omega

Solubility and Dissolution Kinetics of Dolomite in Ca–Mg–HCO3/CO3 Solutions at 25°C and 0.1 MPa Carbon Dioxide - Sherman - 2000 - Soil Science Society of America Journal - Wiley Online Library

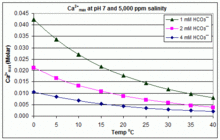
The impact of Mg2+ ions on equilibration of Mg-Ca carbonates in groundwater and brines - ScienceDirect
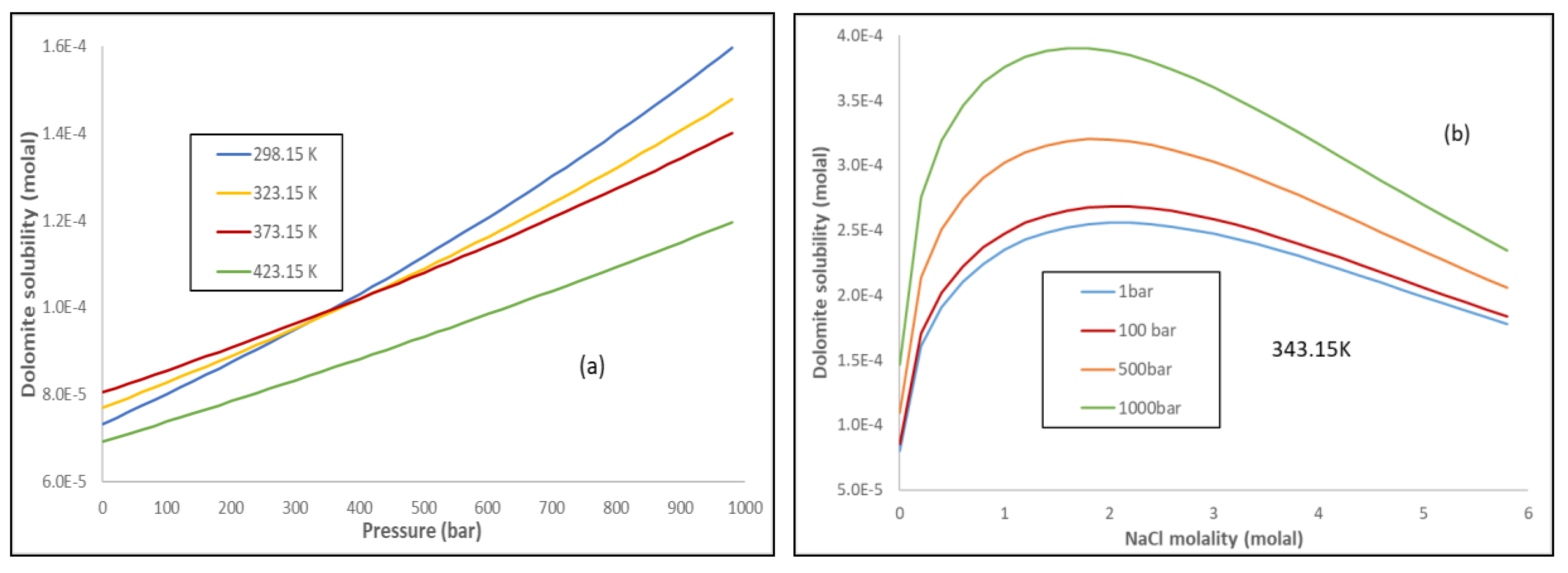
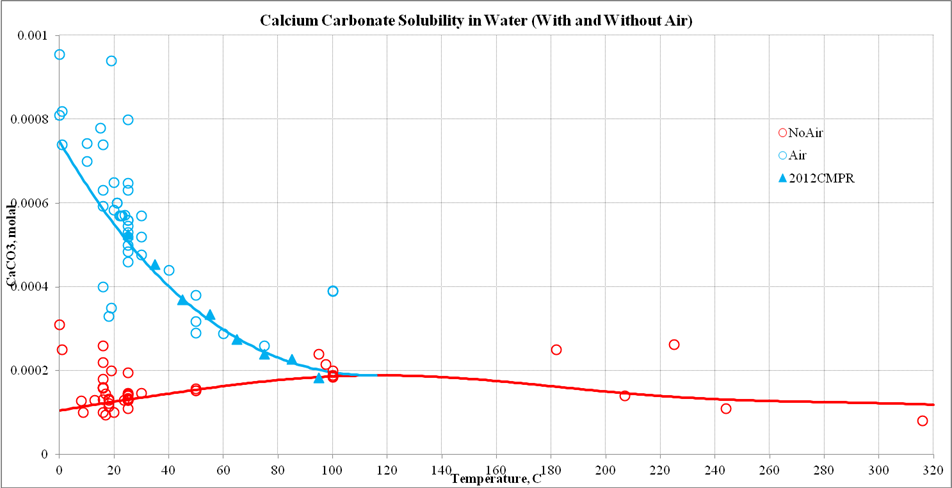
Energies | Free Full-Text | Thermodynamic Modeling of CO2-N2-O2-Brine-Carbonates in Conditions from Surface to High Temperature and Pressure

Low-Temperature Synthesis of Disordered Dolomite and High-Magnesium Calcite in Ethanol–Water Solutions: The Solvation Effect and Implications | ACS Omega

SE - Precipitation of dolomite from seawater on a Carnian coastal plain ( Dolomites, northern Italy): evidence from carbonate petrography and Sr isotopes
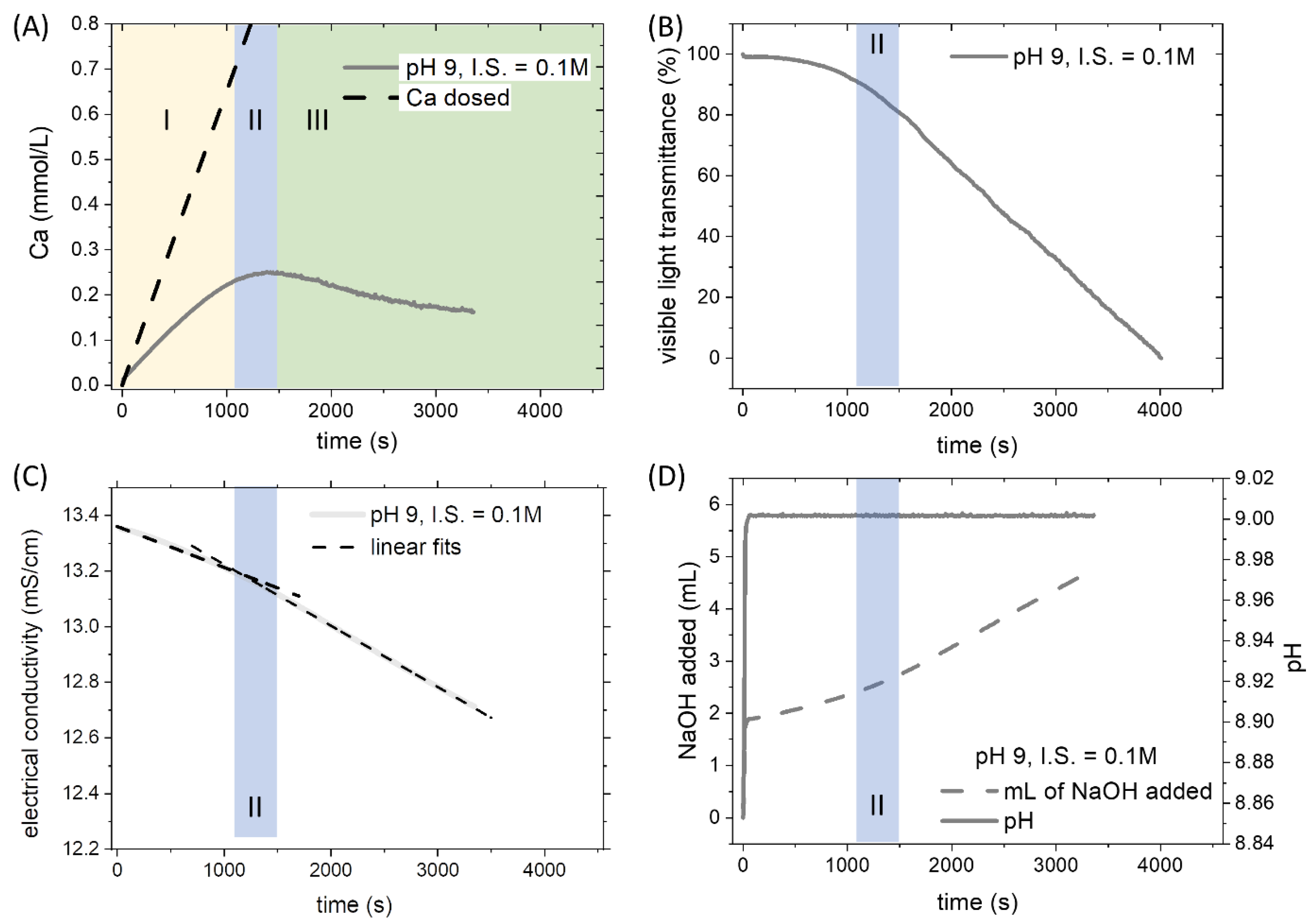
Minerals | Free Full-Text | The Effect of pH, Ionic Strength and the Presence of PbII on the Formation of Calcium Carbonate from Homogenous Alkaline Solutions at Room Temperature

Saturation state of calcite and dolomite as a function of pH in the... | Download Scientific Diagram

Solubility product constants for natural dolomite (0–200 °C) through a groundwater-based approach using the USGS produced water database | American Journal of Science
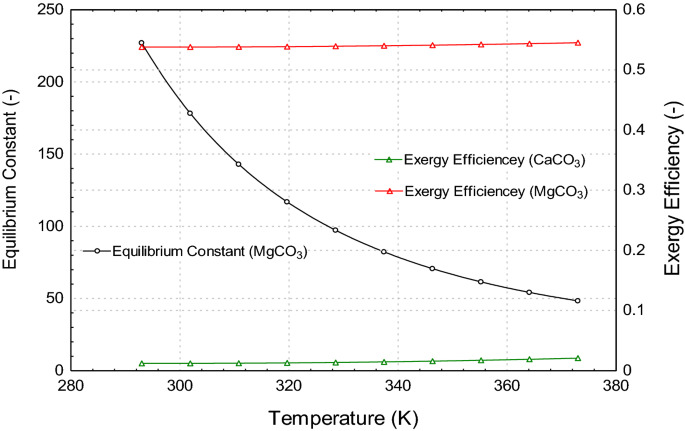
Thermodynamic analysis of theoretical dolomite formation from seawater and captured carbon dioxide | SpringerLink

Solubility and Dissolution Kinetics of Dolomite in Ca–Mg–HCO3/CO3 Solutions at 25°C and 0.1 MPa Carbon Dioxide - Sherman - 2000 - Soil Science Society of America Journal - Wiley Online Library

Greenhouse conditions induce mineralogical changes and dolomite accumulation in coralline algae on tropical reefs | Nature Communications

Self-accelerating volumetric dolomite-for-calcite replacement: A possible mechanism for high-temperature dolomitization? | SpringerLink

Low-Temperature Synthesis of Disordered Dolomite and High-Magnesium Calcite in Ethanol–Water Solutions: The Solvation Effect and Implications | ACS Omega